Abstract
Background:
Advanced Stage Hodgkin lymphoma (HL) is a curable malignancy with combination chemotherapy. While most patients are cured with frontline therapy, for those with refractory disease or early progression, historically, the outcomes have been poor. Novel therapies, PET/CT adapted treatment approaches, and improvement in transplantation have changed the management of both frontline and relapsed HL. However, it remains unknown if these developments have improved the clinical outcomes at population level over time.
Methods:
Using Surveillance Epidemiology and End Results database, we identified patients aged ≥ 18 years with advanced stage (Stage III or IV) pathologically confirmed classical HL as the first primary malignancy,diagnosed between the years 2000-2014, treated with chemotherapy and actively followed. Patients were stratified by date of diagnosis into 3 groups - 2000-2004, 2005-2009, 2010-2014 to assess the trends in overall survival (OS) over time. Race/ethnicity was stratified into non-hispanic whites and minorities (Non-hispanic blacks, Hispanics, other non-hispanic races). Kaplan-Meier method and log rank test were used to analyze the OS among subgroups. Cox proportional hazard regression method was used to determine the influence of period and demographic factors on OS.Cumulative incidence of death from cardiac cause was estimated using the Nelson-Aalen estimates. Statistical analyses were carried out with significant two sided p< 0.05.
Results:
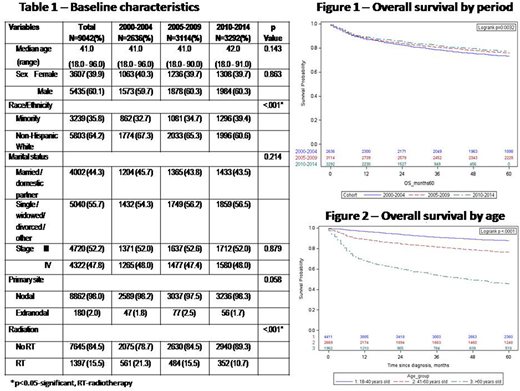
A total of 9042 patients with a median age of 41 years were included. There were more males (60.1%) and non-Hispanic whites (64.2%) and most patients had nodal disease (98%) (Table 1).The use of frontline radiation therapy decreased in each 5-year time period (21.3% 2000-2004 vs 15.5% 2005-2009 vs 10.7% 2010-2014, p<0.001). In terms of survival, when stratified by the period of diagnosis, the 3-year OS was significantly higher for patients diagnosed between the year 2010-2014 (81.8%) and 2005-2009 (80.6%) than those diagnosed from 2000-2004 (78.5%, p=0.0008 and 0.02 respectively) (Figure 1). Additionally, age was a significant predictor for OS with a decreasing 3-year OS with increasing age (age < 40 - 91.1%, age 41-60 -81.5%, age >60 -54%, p< 0.001, Figure 2).While outcomes were poorest in the age>60 cohort, similar improvements were seen in OS over the three time periods among this patient population (48.6%- 2000-2004 vs 54.3% 2005-2009 vs 56.8% 2010-2014, p=0.005). On multivariate analysis, diagnosis in the earlier period was associated with higher mortality (2000-2004-HR 1.36, 95% CI 1.21-1.53, p< 0.001; 2005-2009 -HR 1.14, 95% CI 1.01-1.28, p=0.02, both compared to reference group 2010-2014). Similarly, minority races (HR 1.36, 95%CI 1.23-1.49, p<0.001) had a higher mortality risk as compared to non-Hispanic whites. Females (HR 0.82, 95%CI 0.75-0.90 p<0.001) and married status (HR 0.80, 95%CI 0.72-0.87, p< 0.001) were associated with significantly lower mortality. While radiation use decreased over time, the cumulative incidence of cardiac related cause of death did not vary significantly among the three-time periods (1.2% vs 1.1% vs 1.1% respectively at 48 months, p=0.85).
Conclusions:
Survival of patients with advanced stage HL has continued to improve over time suggesting the clinical impact of evolving treatment approaches. Interestingly this improvement has occurred despite the decreasing utilization of radiation therapy over time. This is suggestive of better end of treatment assessment with PET/CT eliminating the need for end of treatment radiation, improved second line therapies that extend survival, or potentially reduction in treatment related toxicities.Despite these encouraging results, the 3-year OS in the contemporary period remains inadequate at 81.8%. Furthermore, significant differences in survival continue to exist among non-modifiable factors such as gender, age, race, and marital status, highlighting the need for continued research to address these discrepancies. Results of this study provide a new baseline to test novel frontline combination regimens.
Hamadani:Takeda: Research Funding; Celgene Corporation: Consultancy; Cellerant: Consultancy; Ostuka: Research Funding; Janssen: Consultancy; MedImmune: Consultancy, Research Funding; Merck: Research Funding; ADC Therapeutics: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau. Shah:Miltenyi: Other: Travel funding, Research Funding; Exelexis: Equity Ownership; Oncosec: Equity Ownership; Geron: Equity Ownership; Juno Pharmaceuticals: Honoraria; Lentigen Technology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal